 A healthy and intact vascular system in the brain is critical for normal human functioning, both motor and cognitive. Any disruption in the brain’s blood perfusion system can lead to dire consequences, as the brain neurons are deprived of glucose and oxygen and begin to become dysfunctional. An attractive hypothesis that is emerging from a number of medical research groups is that the initiation of certain neurodegenerative diseases, including Parkinson’s disease, occurs when the blood vessels and the blood supply in the brain become compromised. This can occur in a highly localized region of the brain as seen in Parkinson’s disease where in many cases only a very small number of dopamine-producing cells deep in the midbrain are affected. Novel therapeutics which can restore blood flow to damaged areas of the brain leading to neuron regeneration have shown great promise in animal models of brain disorders. One of these therapeutics, human fibroblast growth factor 1 or FGF-1, is now being tested in subjects with Parkinson’s disease utilizing a non-invasive intranasal delivery device to deliver FGF-1 from the nose into the brain.
A healthy and intact vascular system in the brain is critical for normal human functioning, both motor and cognitive. Any disruption in the brain’s blood perfusion system can lead to dire consequences, as the brain neurons are deprived of glucose and oxygen and begin to become dysfunctional. An attractive hypothesis that is emerging from a number of medical research groups is that the initiation of certain neurodegenerative diseases, including Parkinson’s disease, occurs when the blood vessels and the blood supply in the brain become compromised. This can occur in a highly localized region of the brain as seen in Parkinson’s disease where in many cases only a very small number of dopamine-producing cells deep in the midbrain are affected. Novel therapeutics which can restore blood flow to damaged areas of the brain leading to neuron regeneration have shown great promise in animal models of brain disorders. One of these therapeutics, human fibroblast growth factor 1 or FGF-1, is now being tested in subjects with Parkinson’s disease utilizing a non-invasive intranasal delivery device to deliver FGF-1 from the nose into the brain.
Parkinson’s Disease is Initiated by Vascular Dysfunction
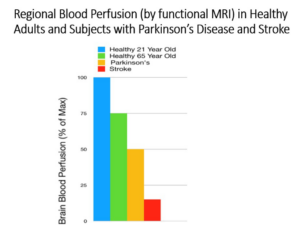 There is a growing body of evidence that Parkinson’s disease is initiated by vascular dysfunction. The hypothesis is that a lack of blood flow initiates the onset of Parkinson’s disease by choking off the dopamine-producing neurons from nourishment and inefficient removal of metabolic wastes that all functioning cells in our bodies produce. Newer imaging technology (functional MRI) can accurately and with high sensitivity determine blood flow in different regions of the brain. As shown in the figure below, a recentstudy looked at blood flow in the region of the brain that is affected in Parkinson’s disease and found that blood flow in this region of the brain decreases a significant 50% in patientswith Parkinson’s disease (orange bar) when compared to healthy 21-year-old’s (blue bar.)
There is a growing body of evidence that Parkinson’s disease is initiated by vascular dysfunction. The hypothesis is that a lack of blood flow initiates the onset of Parkinson’s disease by choking off the dopamine-producing neurons from nourishment and inefficient removal of metabolic wastes that all functioning cells in our bodies produce. Newer imaging technology (functional MRI) can accurately and with high sensitivity determine blood flow in different regions of the brain. As shown in the figure below, a recentstudy looked at blood flow in the region of the brain that is affected in Parkinson’s disease and found that blood flow in this region of the brain decreases a significant 50% in patientswith Parkinson’s disease (orange bar) when compared to healthy 21-year-old’s (blue bar.)
Can Therapeutic Angiogenesis (Stimulating the Growth of New Blood Vessels) Be Used to Treat Neurodegenerative Diseases?
Therapeutic angiogenesis stimulates the growth of new blood vessels where they are required but where they are lacking. In US FDA-cleared clinical trials, therapeutic angiogenesis has been shown to replenish the blood supply to chronic diabetic foot ulcers to speed healing and preventing unnecessary amputations. Other studies have shown that therapeutic angiogenesis can bring healing to oxygen-starved hearts suffering from severe coronary artery disease, allowing “cardiac cripples” to function close to normal again. There is no reason therapeutic angiogenesis could not be applied to Parkinson’s disease and other neurodegenerative disease to reverse and reestablish the growth of blood vessels in the brain, thereby restoring an adequate amount of blood perfusion to allow the brain’s neurons to heal or regenerate.
Fibroblast growth factor 1 (FGF-1), a Potent Stimulator of Angiogenesis
FGF-1, stands out due to its potency and its ability to stimulate the production of not only capillaries, but larger arterioles, which are critical in bringing more blood into the injured tissue. FGF-1 is a natural protein contained within our bodies. It is released upon tissue injury and is the body’s natural response to tissue repair. It has also been shown to be a neurotrophic factor, which is the stimulation of new neuron growth, and it is this characteristic, in addition to its angiogenic properties, that is being exploited for its development as a drug to treat such neurodegenerative diseases as Parkinson’s disease.
FGF-1 Restores Motor Function and Regenerates Dopamine Neurons in a Primate Model of Parkinson’s Disease
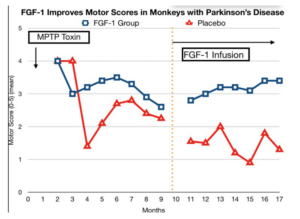 Previous work in animal models of Parkinson’s disease have indicated that growth factors, such as human FGF-1, can restore motor function in both rodent and primate models of this disease. As shown in the figure below, FGF-1 when given over a period of 6 months, substantially improves motor function in monkeys with experimental Parkinson’s disease.The monkeys were given Parkinson’s disease by injecting a toxin into their brains which selectively destroys dopamine-producing cells. Administration of FGF-1 to these animals results in a significant improvement in their motor skills when compared to animals who received a placebo dose as seen in the right-hand side of the figure.
Previous work in animal models of Parkinson’s disease have indicated that growth factors, such as human FGF-1, can restore motor function in both rodent and primate models of this disease. As shown in the figure below, FGF-1 when given over a period of 6 months, substantially improves motor function in monkeys with experimental Parkinson’s disease.The monkeys were given Parkinson’s disease by injecting a toxin into their brains which selectively destroys dopamine-producing cells. Administration of FGF-1 to these animals results in a significant improvement in their motor skills when compared to animals who received a placebo dose as seen in the right-hand side of the figure.
Delivery of FGF-1 to the Brain by an Intranasal Delivery Device
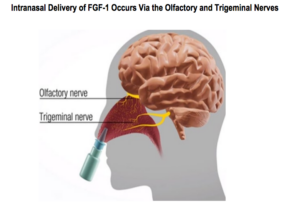
Nose to brain delivery of biological protein drugs, such as FGF-1, can be accomplished with specialized nasal delivery devices which “atomizes” the FGF-1 solution into microscopic droplets (7-micron diameter) and propels the droplets as a gentle vortex high into the nasal chamber where it is then transported into the brain via the olfactory and trigeminal nerves (see figure below). Based upon our calculations, the patients in the first human research study which is now ongoing will receive five times the amount of FGF-1 in their brains than the monkeys received in the successful animal study (figure above) where FGF-1 reversed the progression of their Parkinson’s disease.
 We have collaborated with a research group in Minnesota that has pioneered the intranasal delivery of biologicals such as insulin and FGFs. The results that group obtained with our FGF-1 were genuinely remarkable – almost twice the amount of FGF-1 got into all regions of the brain when comparing intranasal to intravenous dosing of FGF-1.
We have collaborated with a research group in Minnesota that has pioneered the intranasal delivery of biologicals such as insulin and FGFs. The results that group obtained with our FGF-1 were genuinely remarkable – almost twice the amount of FGF-1 got into all regions of the brain when comparing intranasal to intravenous dosing of FGF-1. Dr. Jacobs is President and Chief Science Officer of Zhittya Genesis Medicine, a Las Vegas-based biopharmaceutical company that is developing regenerative biotechnology products through FDA-authorized clinical trials.Don’t miss Dr. Jacobs’ presentation on this topic at t FreedomFest being held in Las Vegas July 13-16, 2022.
Dr. Jacobs is President and Chief Science Officer of Zhittya Genesis Medicine, a Las Vegas-based biopharmaceutical company that is developing regenerative biotechnology products through FDA-authorized clinical trials.Don’t miss Dr. Jacobs’ presentation on this topic at t FreedomFest being held in Las Vegas July 13-16, 2022.
